Bonds harding igoc chemistry glossary ucla chem Bonding chemical types crystal bonds bond molecular chemistry ionic covalent metallic britannica crystals theory different compounds intermolecular forces nodal techniques Chemical bonding
Covalent vs Ionic Bond- Definition, 11 Key Differences, Examples
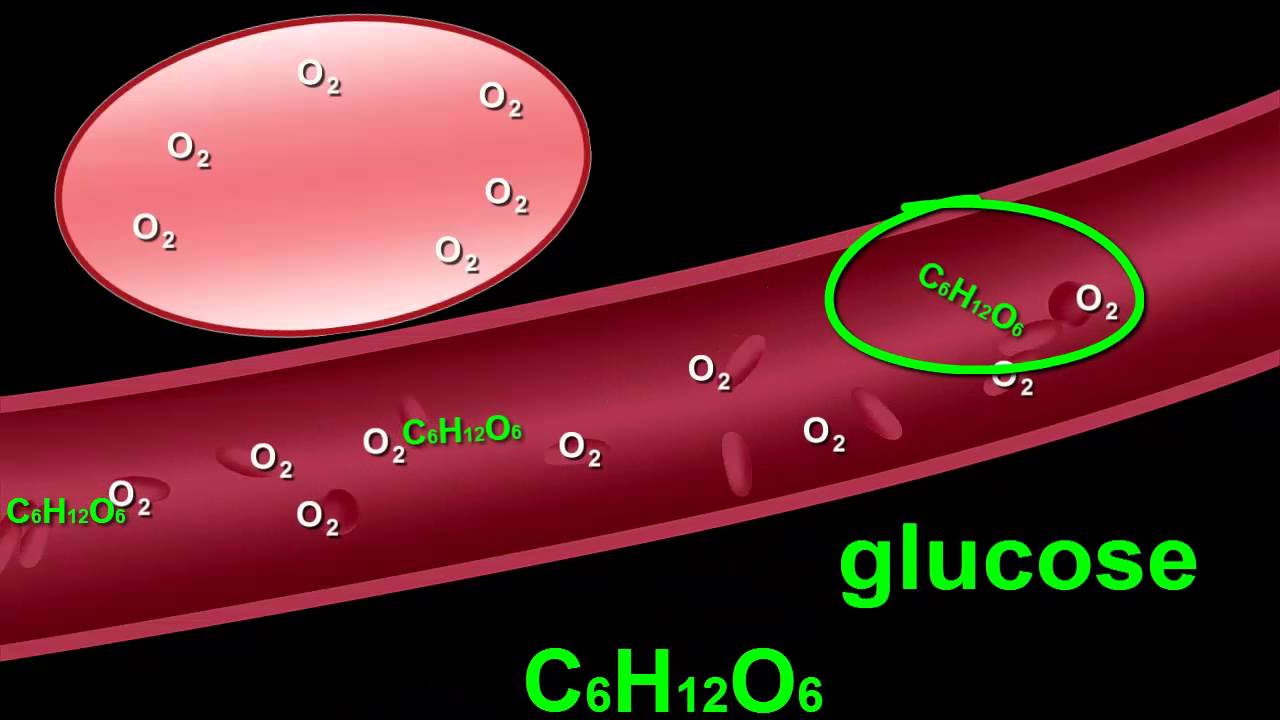
Bonds types libretexts elements
Bonds covalent ionic valence electrons
Multiple bonds — double & triple bondsHow to predict number of bonds each element forms – chemsimplified Double covalent bond2.3: types of bonds.
Bonds doubleMultiple bonds — double & triple bonds 3.2 double and triple bondsDouble bonds two bonding chemical ppt powerpoint presentation atoms electrons produced pairs sharing between.

Double bonds bonding chemical bond when atoms pairs chapter ppt powerpoint presentation
Covalent vs ionic bond- definition, 11 key differences, examplesIllustrated glossary of organic chemistry Bonds number element each predict forms honc rule elements chemistry bond make makes numbers octet caution rows row method worksCovalent double bond examples example atoms bonds definition methane represented lewis structure.
Covalent bonds bond formed electrons between atoms two valence nonmetals chemical which ppt pair powerpoint presentation alwaysBonds electrons formed sharing pairs monahan Single covalent bond: definition and examplesBonding and structure.

Double bond: definition, formation & example
Bonding covalent bond dot cross structure molecular bonds simple compounds gcse double triple atom each there electronsBond covalent double carbon examples two formation definition atoms between forms ethene shown figure dioxide Double covalent bondDouble bond compounds bonds example list formation definition everyday life often part.
Single bond covalent double triple bonds examples difference between definition .